Abstract
Background
Cellular therapies are traditionally manufactured from an apheresis product collected at 'steady state' i.e. without any cytokine administered prior. We sought to evaluate the feasibility of utilising the CD34-negative fraction of recombinant human granulocyte colony stimulating factor (rhG-CSF) mobilised apheresis products for the manufacture of CAR T-cells. The effect of rhG-CSF on CAR T-cell function is largely unknown. We conducted an extensive comparison of phenotypic and functional attributes of CAR T-cells manufactured after rhG-CSF exposure (mobCAR) to those manufactured from a steady state apheresis (ssCAR), in order to test the hypothesis that rhG-CSF mobilised CAR T-cells are functionally equivalent to steady state CAR T-cells.
Methods
Untransduced T-cells (UTD) and CAR T-cells targeting CD33 (CART33) were manufactured using paired steady state (ss) cells and cells collected after rhG-CSF treatment (mob). On a protocol approved by the institutional review board, 4 healthy volunteers had ss cells collected from peripheral blood and cryopreserved, and later underwent an apheresis collection after 4 days of G-CSF (10ug/kg/daily) to collect and cryopreserve mob cells. CART33 were then manufactured from thawed ss and mob cells in paired expansions. Cells were activated using CD3/CD28 beads, transduced with lentiviral vector and expanded using Ex-Vivo based cell culture media (fortified with IL-7/IL-15 and human serum), and cryopreserved when mean cell volume was less than 300fL. Extensive comparisons between ssCART33 and mobCART33 were completed, including analysis by flow cytometry, CyTOF, single cell RNA sequencing (scRNAseq) and metabolomics by mass spectrometry. In vitro assays of degranulation, cytokine production and killing were performed, and in vivo function was assessed using an immunodeficient murine model after engraftment with a CD33 expressing cell line.
Results
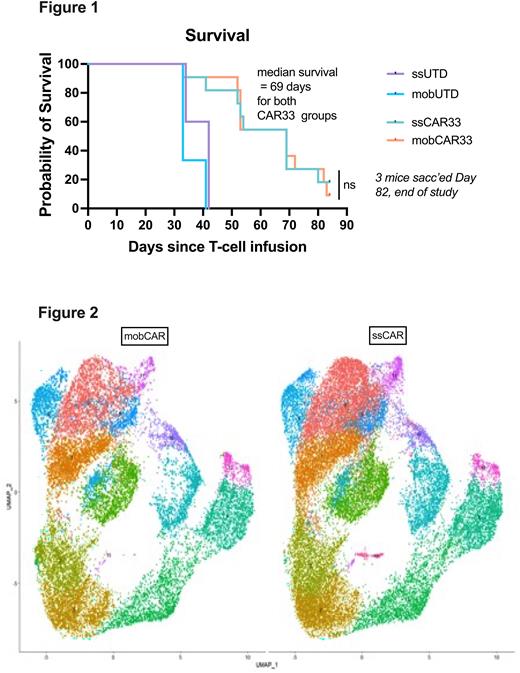
Paired effector T-cells were manufactured with lower fold expansion of mobCAR [4 vs. 7 population doublings, p<0.01] though a sufficient number of mob-CAR were able to be manufactured. Mean CAR expression was higher in ssCAR though this was not statistically significant [ssCAR 78.6% (55-79%) vs. 64.7% (59-71%), p>0.05], and no significant differences in CD4/CD8 ratio were observed. In vitro degranulation, cytokine production and tumour cytolysis were comparable between ssCAR and mobCAR. Functional equivalence was also seen between ssCAR and mobCAR in mouse xenografts of an acute myeloid leukemia (AML) model, with no differences in body weight, toxicity, or mouse survival observed (Figure 1). While significant inter-donor differences in T-cell subsets by CyTOF were observed, minimal differences were seen between the ss and mob products for a given donor, and integrated analysis of scRNASeq data showed a similar transcriptomic profile for ssCAR and mobCAR cells (Figure 2). Metabolomics analysis is ongoing.
Conclusions and future directions
We were able to successfully manufacture CAR T-cell products from G-CSF mobilized cryopreserved apheresis products, and found comparable in vivo anti-tumor efficacy and toxicity, with no significant differences in the G-CSF exposed product by multi-omic analyses. Successful completion of this work may support the use of cryopreserved G-CSF stimulated apheresis units for cell therapy applications. This may allow for an adjunct cell therapy to hematopoietic stem-cell transplant to be manufactured from the same mobilized collection, or provide a means by which previously cryopreserved mobilized units may be utilised, noting frequently lower cumulative chemotherapy exposure in historically cryopreserved T-cells.
Disclosures
Gill:Carisma: Current holder of stock options in a privately-held company, Research Funding; Asher Bio: Research Funding; Interius: Current holder of stock options in a privately-held company, Research Funding; Mission Bio: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria; Immpact Bio: Honoraria; Novartis: Patents & Royalties, Research Funding; Hemogenyx: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal